2025-11-06
Global Stage, New Chapter: Sen

Release Time: 2024-09-03

News Source: PharmaCompass

Author: SENOVA—Lucas
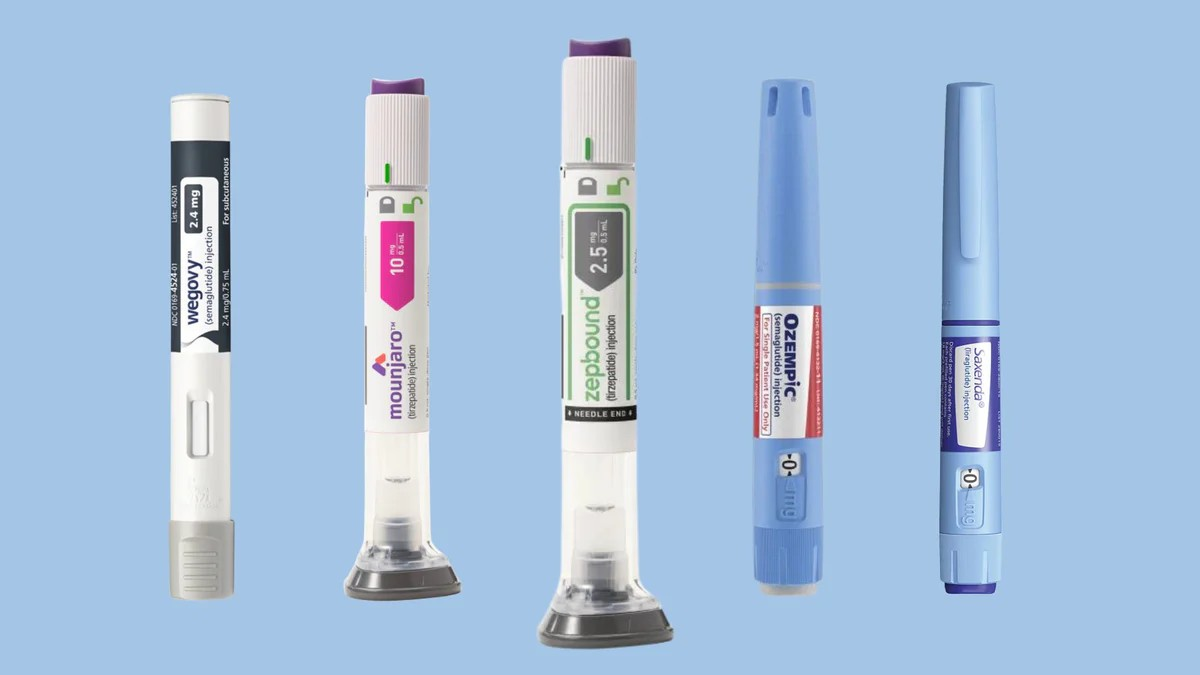
The shortage of glucagon-like peptide-1 (GLP-1) receptor agonists, such as Ozempic, Saxenda, Trulicity, Victoza, Mounjaro, and Zepbound, has been grabbing headlines. These drugs are used in the treatment of diabetes and in weight management.
The shortage of these drugs has led to a surge in demand for compounded versions of these medications, creating both opportunities and significant risks. While the FDA’s Food, Drug, and Cosmetic Act allows it to turn a blind eye to pharmacists producing and selling non-approved compounded versions under certain circumstances, this practice has led to a billion-dollar shadow industry of compounded GLP-1 drugs.
Eli Lilly has discovered compounded drugs advertised as tirzepatide (Mounjaro and Zepbound) with significant safety, sterility, and efficacy issues. In response to the safety risks, Lilly has taken legal action against several wellness centers, medical spas, and other sellers in the US.
To address the supply issues, Lilly has announced its largest manufacturing investment yet, investing an additional US$ 5.3 billion at its Indiana site. Similarly, Novo Nordisk’s parent company is acquiring Catalent for US$ 16.5 billion to increase Wegovy production. Novo is also investing US $ 4.1 billion to develop a new manufacturing facility in Clayton, North Carolina (US).
The FDA website continues to list Novo and Lilly’s GLP-1 drugs as “Currently in Shortage”, even though the drugmakers say otherwise. The EMA website says the shortage of Novo’s Wegovy has been resolved but Lilly’s Trulicity and Novo’s Saxenda continue to be in shortage.