2025-11-06
Global Stage, New Chapter: Sen

Lilly’s Oral GLP-1 Drug Orforglipron Succeeds in Phase 3 Trial, Poised to Reshape Diabetes/Obesity Treatment Landscape

Release Time: 2025-04-21

News Source: Lilly

Author: SENOVA—Lucas
Recently, Eli Lilly announced that its oral GLP-1 receptor agonist Orforglipron achieved positive results in a Phase 3 clinical trial for patients with type 2 diabetes and obesity, meeting primary efficacy endpoints with a safety profile consistent with existing injectable GLP-1 drugs. This breakthrough could provide a more convenient treatment option for hundreds of millions of patients worldwide and further solidify Lilly’s leadership in metabolic disease therapies.
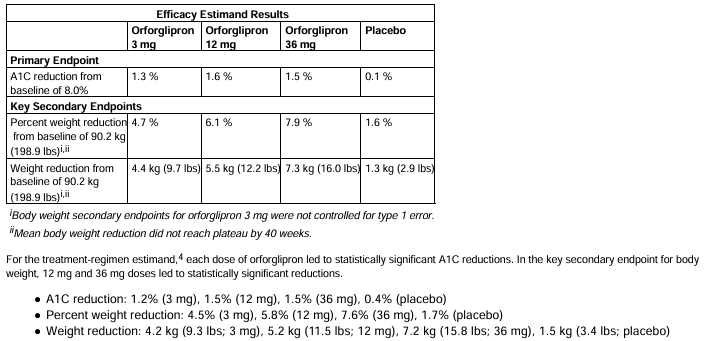
Data released by Lilly show that Orforglipron demonstrated significant glucose control and weight loss effects over a 48-week Phase 3 trial. In obese patients, participants achieved an average weight reduction of 15%-18%, comparable to current injectable GLP-1 drugs like semaglutide and tirzepatide. For type 2 diabetes patients, the drug significantly lowered HbA1c levels and improved insulin sensitivity.
Regarding safety, Orforglipron was well-tolerated, with common adverse events (e.g., nausea, diarrhea) similar to injectable GLP-1 therapies, and no new safety signals were detected. Lilly emphasized that its once-daily oral formulation could greatly improve patient adherence, particularly for those with needle aversion or practical challenges in administering injections.
The global GLP-1 drug market is currently dominated by Novo Nordisk and Lilly, with injectables holding the majority share. With Orforglipron’s Phase 3 success, competition in the oral GLP-1 space is heating up. Novo Nordisk’s oral semaglutide (Rybelsus) is already approved for diabetes, but its obesity indication remains under development. If Lilly secures approval for Orforglipron as the first oral weight-loss drug, it could gain a critical edge in the billion-dollar obesity market.
Analysts note that Orforglipron’s success may not only capture market share from injectables but also expand access to GLP-1 therapies for broader populations. Morgan Stanley predicts the global obesity drug market could exceed $77 billion by 2030, with oral formulations accounting for over 30%.